Current Issues
Original Article
Vascular loops in the cerebellopontine angle cistern and their role in causing compression symptoms of 7th and 8th nerves as determined in Magnetic Resonance Imaging
*Corresponding Author
Kusuma Kumar Kota
Senior resident, Department of Radiology,
Kamineni Institute of Medical Sciences, Narketpally, Nalgonda district, Telangana state, India – 508 254,
Email Id: kota_kusum@yahoo.co.in
1Senior resident, 2Assistant Professor, 3Professor and Head, Department of Radiology, 4Assistant professor, Department of Neurosurgery, Kamineni Institute of Medical Sciences, Narketpally, Nalgonda District, Telangana State, India.
Abstract
Introduction: Neurovascular compression syndrome is a group of symptoms seen as a result of compression or irritation of the nerve root by aberrant or tortuous vessel. The vessels in the cerebellopontine (CP) angle are classified into four types depending on their extension into the CP angle and formation of a loop. Neurovascular conflict (NVC) is defined as an "abnormal" contact between a vessel and a cranial nerve. Three-dimensional constructive interference in steady state (CISS) is routinely used in the assessment of cerebellopontine angle and its fine structures like 7th and 8th cranial nerves.
Aim: To identify the most common type of vasculature in the cerebellopontine angle and the most common type implicated in neurovascular compression by identifying the conflict rather than just contact of the vessel with the nerve.
Materials and methods: 100 patients (with 200 sides) were taken for scanning with defined criteria for study purpose. Out of them, 64 patients had unilateral symptoms, the symptomatic side was taken as diseased and asymptomatic side was taken as controls. 18 patients with bilateral symptoms were taken as diseased and 18 patients with no symptoms of cranial nerve compression were taken as controls. Out of the study subjects, 64 patients were with unilateral symptoms, 18 patients with bilateral symptoms and 18 patients with no symptoms of cranial nerve compression (A total of 100 sides diseased and 100 sides controls were studied). Most common type causing neurovascular conflict was studied and compared between cases and controls and the statistical significance of which was assessed using Chi square test.
Results: Most common pattern of vascularity in all cases together was type IA (44%), in cases with 7th nerve compression symptoms is type IA (82%) and in cases with 8th nerve compression symptoms was type IB (50%). Neurovascular contact noted in 97% cases with 7th nerve compression symptoms out of which there was conflict in 88.2%. In cases with 8th nerve compression symptoms, neurovascular contact was noted in 65.15% and conflict noted in 27.27%. This difference was statistically significant. Conclusion: Most common vascular pattern is type IA in cases, type IB in controls and type IB in cases and controls together. Vascular type implicated in 7th nerve compression was type IA and there is no statistically significant relation between the vascular pattern and nerve compression in 8th nerve.
Key words:Neurovascular Compression, Neurovascular Conflict, 7th and 8th Nerves, Vascular Loops
Introduction
Neurovascular compression syndrome is a group of symptoms seen as a result of compression or irritation of the nerve root by aberrant or tortuous vessel.1
7th and 8th nerves exit the brain stem and enter the internal auditory canal (IAC) after passing through the cerebellopontine (CP) angle cistern where they come in close contact with the anterior inferior cerebellar artery or the superior cerebellar artery.2 Compression of 7th nerve at the root entry zone is known to cause primary hemi facial spasms. Vertigo, tinnitus and sensorineural hearing loss have different etiological factors, however, 8th nerve compression is one of the causative factor.
The vertebrobasilar system is composed of longitudinal vessels with a myriad transverse vessels, each having the potential to become superior cerebellar artery (SCA) or anterior inferior cerebellar artery (AICA) by capturing the cortical territory of the developing cerebellum. Thus many variants of the posterior inferior cerebellar artery (PICA), AICA and SCA have been seen as a normal physiology of development.3
Anatomical classification of the vessels in the CP angle done by Kazawa et al4 in 2013 was used in the present study. The vessels are classified in to four types, i.e. Type IA, IB, IIA and IIB.
Three-dimensional (3D) Constructive Interference in Steady State (CISS) is a fully refocused steady-state gradient-echo MRI sequence. Image contrast in CISS is determined by the T2/T1 ratio of the tissue, which gives clear imaging of all the cranial nerves. Three-dimensional CISS is routinely used in the assessment of cerebellopontine angle and its fine structures like 7th and 8th cranial nerves and membranous labyrinth. CISS images can be acquired in any plane but most commonly in the axial plane, for cranial nerve imaging.4
Neurovascular conflict (NVC) is defined as an "abnormal" contact between an artery and the cranial nerve.5 So, it was proposed to study the types of vasculature in the cerebellopontine angle cistern and their role in causation of symptoms of 7th and 8th nerve compression.
Aims and Objectives
Aim: To study the role of vascular loops in the CP angle in causing symptoms of compression of 7th and 8th cranial nerves, as determined in MRI.
Objectives
- To study the most common type of vascular loop and to identify the type implicated in compression of nerves.
- To identify the role of neurovascular conflict in causation of symptoms rather than just contact of the vessel with the nerve.
Materials and Methods
- Type of study: Prospective case control study.
- Place of study: Kamineni Institute of Medical Sciences, Narketpally.
- Duration of study: From January 2015 to November 2015 (11 months).
Definition of cases and controls:
- Cases: Patients of all ages and both sexes with vertigo, tinnitus, hemi facial spasms or other symptoms related to 7th and 8th nerve compression, referred to the department of radiodiagnosis for MRI brain.
- Controls: Patients of all ages and both sexes with symptoms other than those caused by 7th and 8th nerve compression, referred to the department of radiodiagnosis for MRI brain.
Sample size:
- Cases: 82 patients (100 sides) - 64 patients with unilateral symptoms (64 sides) and 18 patients with bilateral symptoms (36 sides).
- Controls: 82 patients (100 sides) – 64 patients with asymptomatic sides of cases (64 sides) and 18 patients with no symptoms of 7th and 8th nerve compression (36 sides).
Inclusion criteria
- Cases: Patients with 7th and 8th nerve compression symptoms.
- Controls: Patients with symptoms other than those caused by 7th and 8th nerve compression.
Exclusion criteria
- Cases: Known cases of CP angle masses, cases with fluid signal in middle ear or mastoid, posterior circulation stroke, or any finding other than vascular loop causing symptoms of 7th and 8th nerve compression, patients presenting in emergency and patients in whom MRI is contraindicated are excluded from the study.
- Controls: Patients presenting in emergency and cases with contraindications for MRI.
Methodology
- Institutional ethics committee permission was taken before starting the study.
- Informed written consent was taken from the cases and controls in the language understandable by patient.
- The selected patients were scanned with the regular and required sequences initially (T2 axial, FLAIR axial, T1 sagittal, DWI & SWI axial sequences) using 1.5 tesla Siemens Magnetom Essenza machine.
- Additional sequence (CISS – Constructive interference in steady state) performed in all the cases and controls.
- Vessels in CP angle cistern were observed and classified as follows.
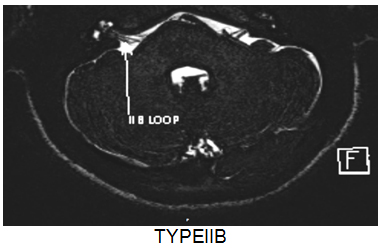
- Type IA – Non loop type in CP angle cistern.
- Type IB – Loop type in CP angle cistern.
- Type IIA – Non loop type, entering the internal auditory canal.
- Type IIB – Loop type, entering the internal auditory canal.
- Types of loops in CP angle cistern were tabulated in cases and controls & according to various symptoms.
- Most common pattern in the cases, for each symptom was identified and compared with of controls. The statistical significance was assessed using chi square test.
- Neurovascular conflict was identified basing on the following factors:5
- Presence of neurovascular contact
- Orthogonal contact between the nerve and the vascular loop
- Angulation of the nerve at the site of contact
- Site of contact of the nerve and the vascular loop (root entry zone, cisternal segment or canalicular segment)
- The cases and controls showing neurovascular contact and conflict were tabulated. Statistical significance of neurovascular conflict was identified using the Chi square test.
Results
A total of 100 patients were scanned with 1.5 Tesla MRI. Cases and controls were age and sex matched, with average age being 44 years for cases and 43 years for controls.
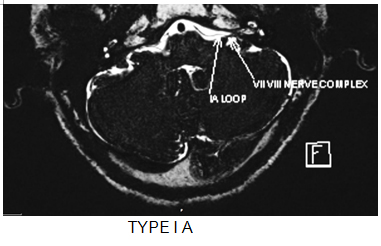 Fig .1 : MRI images of different types of vascular loops in cerebellopontine angle cistern.
Fig .1 : MRI images of different types of vascular loops in cerebellopontine angle cistern.
 Out of 64 patients with unilateral symptoms, 34 cases presented with Hemi facial spasms, indicating 7th nerve compression. 20 with unilateral tinnitus, 10 with unilateral sensorineural hearing loss indicating 8th nerve compression.
Out of 64 patients with unilateral symptoms, 34 cases presented with Hemi facial spasms, indicating 7th nerve compression. 20 with unilateral tinnitus, 10 with unilateral sensorineural hearing loss indicating 8th nerve compression.
Out of 18 patients with bilateral symptoms, 2 patients presented with bilateral tinnitus, 2 with sensorineural hearing loss and 14 with vertigo.
64 cases with unilateral symptoms suggestive of 7th and 8th nerve compression, the normal side is considered as control.
18 cases who presented with symptoms other than 7th and 8th nerve compression are considered as controls for rest of the sides – 36 sides.


Most common pattern in cases is type IA (n=44) (44%) and controls is IB (n=33) (33%) as shown in the bar diagram (Fig 3) and this difference was found to be statistically significant as calculated by Chi square test (Chi sq value = 27.359, df = 3, p value < 0.01). However, most common type in cases and controls together is type IB (n=68) (34%)(Table 1).
Fig. 3 : Type of vasculature in cases and controls
Table 1: Type of vasculature in cases and controls of 7th nerve compression symptoms (N=68)
| Type of vessel | Cases n(%) | Controls n(%) | Total n(%) |
| IA | 28 (82.3) | 9(26.5) | 37(54,4)* |
| IB | 2(5.9) | 14(41.2) | 16(23.5) |
| IIA | 2(5.9) | 5(14.7) | 7(10.3) |
| IIB | 2(5.9) | 6(17.6) | 8(11.8) |
| Total | 34(100) | 34(100) | 68(100) |
* Statistically significant, p value<0.01
Most common pattern in cases with 7th nerve compression symptoms is type IA (n=28) and controls is IB (n=14) and this difference was found to be statistically significant as calculated by Chi square test (Chi sq = 20.802, df=3, p value <0.01). However, the most common type among the loops in cases and controls together was type IA (n=37) (Table 2).
Table 2: Type of vasculature in cases and controls of 8TH nerve compression symptoms (n=132)
| Type of vessel | Cases n(%) | Controls n(%) | Total n(%) | |
| IA | 16(24.3) | 7(10.6) | 23(17.5)* | |
| IB | 33(50) | 21(31.8) | 54(40.9) | |
| IIA | 9(13.6) | 16(24.2) | 25(18.9) | |
| IIB | 8(12.1) | 22(33.4) | 30(22.7) | |
| Total | 66(100) | 66(100) | 132(100) | |
* Statistically significant p value <0.01
Most common pattern in cases with 8th nerve compression symptoms is type IB (n=33) and controls is IIB (n=22) and this difference was found to be statistically significant as calculated by Chi square test (Chi Sq = 14.682, df=3, p value <0.01). However, the most common type among the loops in cases and controls together is type IB (n=54) (Table 2).
Neurovascular conflict: Presence of neurovascular conflict is identified by the following criteria5
- Presence of neurovascular contact
- Orthogonal contact between the nerve and the vascular loop
- Angulation of the nerve at the site of contact
- Site of contact of the nerve and the vascular loop (root entry zone, cisternal segment or canalicular segment)
Neurovascular contact noted in 33 cases out of 34 symptomatic patients (97%) out of which there is neurovascular conflict in 30 cases (88.2%), whereas neurovascular contact noted in17 out of 34 controls (50%) and in these, there is neurovascular conflict in 4 controls (11.8%). This difference was found to be statistically significant with (Chi sq = 5.336) p value 0.02. (Table 3)
Table 3: Neurovascular contact and conflict in cases and controls of 7th nerve compression
| Neuro-vascular relation | Cases n(%) (n=34) | Controls n(%) (n=34) | |
| Contact | Yes | 33 (97) | 17 (50) |
| No | 1 (3) | 17 (50) | |
| Conflict | Yes | 30 (88.2) | 4 (11.8) |
| No | 4 (11.8) | 30 (88.2) |
* Statistically significant p value <0.05
Neurovascular contact noted in 43 cases out of 66 symptomatic patients (65.15%) out of which there was neurovascular conflict in 18 cases (27.27%), whereas neurovascular contact noted in 33 out of 66 controls (50%) and in these, there was neurovascular conflict in 6 controls (9.09%). This difference was not statistically significant with p value 0.106. (Table 4)
Table 4: Neurovascular contact and conflict in cases and controls of 8th nerve compression
| Neuro-vascular relation | Cases n(%) (n=66) | Controls n(%) (n=66) | |
| Contact | Yes | 43 (65.2) | 33 (50) |
| No | 23 (34.8) | 33 (50) | |
| Conflict | Yes | 18 (27.3) | 6 (9.1) |
| No | 48 (72.7) | 60 (90.9) |
* Statistically significant p value <0.05
Our study showed that symptomatic patients had as much neurovascular contact of the 8th cranial nerve in cerebellopontine angle as the asymptomatic patients had but no statistically significant neurovascular conflict was found.
Discussion
Neurovascular compression is considered as one of the etiologic factor for symptoms of 8th nerve compression and the main etiologic factor in 7th nerve compression symptoms.
Vascular compression syndromes of the cranial nerves, first suggested in 1934 by Dandy and then popularized by Jannetta in the 1970s,7 are gaining acceptance with the improvement in MRI assessment and with the success of endoscopy-assisted microvascular decompression procedures.
MRI is able to reproduce the anatomy of the CP angle and to show the presence of neurovascular conflict. Imaging of the CP angle was carried out with serial thin slices of 1 mm thickness sections Three dimensional CISS. The axial plane offers the most comprehensive images.4 Three-dimensional CISS is helpful in visualizing faint structural elements in the central nervous system because of its higher spatial resolution and fewer artifacts from the CSF and it is used to investigate a wide range of pathologies when routine MRI sequences do not provide the desired anatomic information.8
CISS sequence is able to classify the vascular loops in cerebellopontine angle cistern as IA, IB, IIA and IIB.
In the present study, Most common pattern in cases was type IA (44%) and controls was IB (33%) and, most common type in cases and controls together was type IB (34%). In the study by N. Kazawa et al,4 most common type in cases was type IIA (37%), controls was type IA (61%), most common in cases and controls together was type IA (46.5%).
Most common pattern in cases with 8th nerve compression symptoms was type IB (50%), controls was IIB (34%) and most common type among cases and controls together was type IB (40.9%). In the study by N. Kazawa et al4., most common type in cases was type IIA (40.1%), controls was type IA (57%), most common in cases and controls together was type IIA (38%).
Most common pattern in cases with 7th nerve compression symptoms was type IA (82%) and controls was IB (41%) the most common type among the loops in cases and controls together was type IA (54%).
The difference between the studies is thought to be because of smaller sample size in their study (55) as compared to the present study (100 patients).
Root entry zone (REZ) is the cisternal part of the nerve close to the entrance into the pons (or the exit from). It represents a transition zone between the peripheral myelin, derived from Schwann cells, and central myelin, derived from oligodendroglia. According to this anatomical organization, this junctional zone is thinner and more vulnerable to vascular compression.9
Distortion of the nerve course by the vascular loop causes stretching of the nerve, which weakens the nerve; also, compression of the adjacent delicate neural structure (brain stem) at the REZ has been found to be a cause.
For the facial nerve, the location of the conflict at the REZ which is located 8mm away from pons.8
In the present study, neurovascular contact and conflict were evaluated using CISS sequence of the cerebellopontine angle and concluded that, though there was statistically significant contact between the nerves and the vessels, there has been a failure in determining the significance of the vessel in causing symptoms of 8th nerve compression, when the neurovascular conflict criteria are considered.10
In 7th nerve compression patients, it was observed that 33 had contact and 30 had conflict. In 8th nerve compression patients, 43 had contact and 18 had conflict.
Conclusion
- In the present study we described various vascular loop types in cerebellopontine angle and their role in compression of cranial nerves leading to various symptoms like hemi facial spasms, vertigo, tinnitus.
- Most common pattern in cases was type IA and controls was IB and most common type in cases and controls together was type IB.
- Most common pattern in cases with 7th nerve compression symptoms was type IA, controls was IB and the most common type among the loops in cases and controls together was type IA.
- Most common pattern in cases with 8th nerve compression symptoms was type IB, controls was IIB and most common type among the loops in cases and controls together was type IB.
- There was statistically significant relation between the neurovascular conflict and symptoms in 7th nerve cases and no statistically significant relation between neurovascular conflict and the symptoms in 8th nerve cases.
References
- Sherif Elaini, Jaques Magnan, Arnaud Deveze. Endoscopic criteria of offending vessel in neurovascular compression syndrome. The Egyptian Journal of Otolaryngology 2014, 30:305–310.
- Régis J, Roche P-H. Modern Management of Acoustic Neuroma. Prog Neurol Surg. 2008, 21: 43-53.
- 3 Anterior inferior cerebellar artery (AICA) [Internet] 2015 Available from: http://neuroangio.org/anatomy-and-variants/aica/
- Kazawa, Nobukata; Togashi, Kaori; Ito, Juichi The anatomical classification of AICA/PICA branching and configurations in the cerebellopontine angle area on 3D-drive thin slice T2WI MRI. Clinical imaging 2013, 37(5): 865-870.
- Sherif Elainia, Jaques Magnanb, Arnaud Devezeb and Nadine Girardc Magnetic resonance imaging criteria in vascular compression syndrome The Egyptian Journal of Otolaryngology 2013, 29:10–15.
- E. Lozupone, G. Di lella, S. Gaudino, A. Pedicelli, R.Colantonio, M. Martucci, M. Pileggi, L. Bonomo, C. Colosimo. Imaging neurovascular conflict: what a radiologist need to know and to report?. ecr2012/C-1933.
- Jannetta PJ. Microsurgery of cranial nerve cross-compression. Clin Neurosurg 1979; 26:607–615.
- Yang D, Korogi Y, Ushio Y, Takahashi M. Increased Conspicuity of Intraventricular Lesions Revealed by Three-dimensional Constructive Interference in Steady State Sequences.Am J Neuroradiol. 2000;2:1070–2.
- Sudipta Saha , Sumit Chakraborthy, Surojit Das, Avik Sarkar, Akash Mukherjee. MR evaluation of neurovascular conflict. PJR October- December 2014; 24(4): 147-150.
- S. Gultekin, H. Celik, S. Akpek, Y. Oner, T. Gumus, N. Tokgoz. Vascular Loops at the Cerebellopontine Angle: Is There a Correlation with Tinnitus? AJNR Am J Neuroradiol Oct 2008. 29:1746–49


